Preserving health
and quality of life

More cancer patients than ever before experience initial treatment success, leading to clinical remission of the disease. However, tumor recurrence due to residual cancer cells is an imminent threat and responsible for the majority of cancer-related deaths.
Mendus addresses tumor recurrence by developing therapies that enable the immune system to build up active immunity against residual cancer cells, in order to improve long-term survival while preserving health and quality of life.
Our Approach
Addressing unmet needs in cancer treatment
Acute Myeloid leukemia (AML)
Disease relapse following initial treatment is the main barrier to long-term survival in AML. Particularly, measurable residual disease, or MRD, is associated with increased relapse risk and poorer overall survival.
Following positive Phase 2 survival data from the ADVANCE II trial exploring Mendus’ lead program vididencel as a maintenance therapy for AML patients diagnosed with MRD, Mendus is expanding clinical development and preparing vididencel for pivotal-stage development in AML.
Ovarian Cancer
Ovarian cancer is the deadliest gynecological malignancy, due to its high recurrence rate following first line surgery and chemotherapy.
The Phase 1 ALISON trial evaluates vididencel safety and efficacy in ovarian cancer. If successful, the trial opens up the potential development of vididencel as a novel maintenance treatment in this indication.
Intratumoral immune priming
The intratumoral immune primer ilixadencel has demonstrated signs of clinical efficacy in a range of hard-to-treat solid tumors, including renal cell carcinoma (RCC), hepatocellular carcinoma (HCC) and gastro-intestinal stromal tumors (GIST). It also has a good safety profile in combination with other cancer drugs, such as tyrosine kinase inhibitors and immune checkpoint inhibitors, providing a potential novel combination treatment option for tumors poorly responding to currently available therapies.

Pipeline
Vididencel
AML (monotherapy)
Maintenance therapy for patients with measurable residual disease
Study
ADVANCE IIStatus
Ongoing (long-term follow-up)
- Preclinical
- Phase 1
- Phase 2
- Pivotal
AML (with oral azacitidine)
Maintenance therapy in combination with oral AZA, collaboration with Australasian Leukaemia & Lymphoma Group (ALLG)
Study
AMLM22-CADENCEStatus
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Ovarian Cancer
Maintenance therapy in combination with standard of care, collaboration with University Medical Center Groningen (UMCG)
Study
ALISONStatus
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Ilixadencel
Hard-to-treat solid tumors
Multiple completed clinical trials
Status
Subject to partnering
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Preclinical
NK cell platform
Method for expansion of memory NK cells
Status
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Technology
Mendus applies dendritic cell biology to design off-the-shelf immunotherapies that enable the body’s own immune system to build up active, long-lasting immunity against tumor cells.

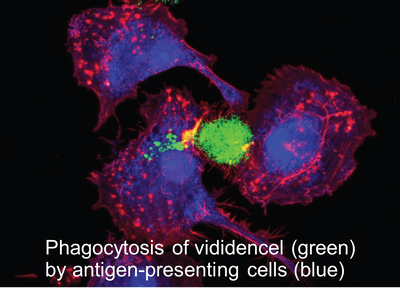
Vididencel comprises irradiated leukemic-derived dendritic cells derived from a proprietary leukemic cancer cell line (DCOne). Upon intradermal injection, vididencel is phagocytosed by antigen-presenting cells present in the skin, which subsequently trigger broad immune responses against the tumor antigens carried by vididencel.
The intratumoral immune primer ilixadencel comprises pro-inflammatory dendritic cells from healthy donors, which are administered directly into the tumor. This leads to local inflammation in the tumor microenvironment and the triggering of anti-tumor immune responses.
Our preclinical pipeline focuses on the use of the DCOne platform for ex vivo expansion of immune cells for therapeutic purposes and the exploration of novel combination therapies.
Publications
Patient Stories
Patient stories providing insight into medical needs, treatment experiences, and everyday challenges of living with cancer

Giora Sharf’s 25-year history managing CML
“Don’t stop innovating—patients still need better, safer options.”

Jacob’s AML journey
“Ann and I exercise every day together, taking walks in the forest or at the gym. We take this day by day and with our first grandchild set to join us in August we have a lot to look forward to.”

