Preserving health
and quality of life

More cancer patients than ever before experience initial treatment success, leading to clinical remission of the disease. However, tumor recurrence due to residual cancer cells is an imminent threat and responsible for the majority of cancer-related deaths.
Mendus addresses tumor recurrence by developing therapies that enable the immune system to build up active immunity against residual cancer cells, in order to improve long-term survival while preserving health and quality of life.
Our Approach
Addressing unmet needs in cancer treatment
Acute Myeloid leukemia (AML)
Disease relapse following initial treatment is the main barrier to long-term survival in AML. Particularly, measurable residual disease, or MRD, is associated with increased relapse risk and poorer overall survival.
Following positive Phase 2 survival data from the ADVANCE II trial exploring Mendus’ lead program vididencel as a maintenance therapy for AML patients diagnosed with MRD, Mendus is expanding clinical development and preparing vididencel for pivotal-stage development in AML.
Ovarian Cancer
Ovarian cancer is the deadliest gynecological malignancy, due to its high recurrence rate following first line surgery and chemotherapy.
The Phase 1 ALISON trial evaluates vididencel safety and efficacy in ovarian cancer. If successful, the trial opens up the potential development of vididencel as a novel maintenance treatment in this indication.
Intratumoral immune priming
The intratumoral immune primer ilixadencel has demonstrated signs of clinical efficacy in a range of hard-to-treat solid tumors, including renal cell carcinoma (RCC), hepatocellular carcinoma (HCC) and gastro-intestinal stromal tumors (GIST). It also has a good safety profile in combination with other cancer drugs, such as tyrosine kinase inhibitors and immune checkpoint inhibitors, providing a potential novel combination treatment option for tumors poorly responding to currently available therapies.

Pipeline
Vididencel
AML (monotherapy)
Maintenance therapy for patients with measurable residual disease
Study
ADVANCE IIStatus
Ongoing (long-term follow-up)
- Preclinical
- Phase 1
- Phase 2
- Pivotal
AML (with oral azacitidine)
Maintenance therapy in combination with oral AZA, collaboration with Australasian Leukaemia & Lymphoma Group (ALLG)
Study
AMLM22-CADENCEStatus
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Ovarian Cancer
Maintenance therapy in combination with standard of care, collaboration with University Medical Center Groningen (UMCG)
Study
ALISONStatus
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Ilixadencel
Hard-to-treat solid tumors
Multiple completed clinical trials
Status
Subject to partnering
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Preclinical
NK cell platform
Method for expansion of memory NK cells
Status
Ongoing
- Preclinical
- Phase 1
- Phase 2
- Pivotal
Technology
Mendus applies dendritic cell biology to design off-the-shelf immunotherapies that enable the body’s own immune system to build up active, long-lasting immunity against tumor cells.

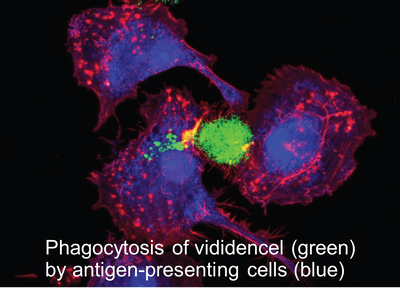
Vididencel comprises irradiated leukemic-derived dendritic cells derived from a proprietary leukemic cancer cell line (DCOne). Upon intradermal injection, vididencel is phagocytosed by antigen-presenting cells present in the skin, which subsequently trigger broad immune responses against the tumor antigens carried by vididencel.
The intratumoral immune primer ilixadencel comprises pro-inflammatory dendritic cells from healthy donors, which are administered directly into the tumor. This leads to local inflammation in the tumor microenvironment and the triggering of anti-tumor immune responses.
Our preclinical pipeline focuses on the use of the DCOne platform for ex vivo expansion of immune cells for therapeutic purposes and the exploration of novel combination therapies.
Publications
Patient and Clinical Perspectives
Patient stories and reflections from leading researchers and experts in immuno-oncology, providing insight into medical needs, treatment experiences and emerging therapeutic advances.

Mendus presented updated survival data from the ADVANCE II Phase 2 trial during the American Society of Hematology (ASH) conference last December, which showed that the majority (13/20) of patients treated with vididencel were alive at a median follow-up of 41.8 months, with 11 out of the 20 patients still in first complete remission, without relapse. To hear first-hand what vididencel treatment can mean for AML patients, Mendus Director Clinical Operations Annelies Legters had a meeting in Bergen, Norway with Jacob, one of the participants in the ADVANCE II trial.
Jacob’s coughing started in March 2017 and by August it was continuous and now severe backpain and night sweats were making him miserable. He already struggled with heart and lung issues, but his health continued to decline further, and he became very sick. His wife, Ann, suspected something was seriously wrong and joined him at the doctor’s in Norway. Days after the appointment, Jacob was diagnosed with acute myeloid leukemia (AML). Due to his lung fibrosis, he was kept in a coma for six weeks during the first round of chemo.
With the first round over, he slowly woke and could only move his eyes, communicating with Ann and the medical team by blinking during the following months. A half year later he moved to rehab to regain some of the 20kg he had lost and to learn to walk and eat again. But with Jacob still battling AML, there were few options but to try a second round of chemo. This time he would undergo the treatment at home with Ann managing his care for the following months. Their children wore protective clothing when visiting to reduce the chance of infection.
“Going through chemo to fight my AML was incredibly difficult because of the painful side effects,” said Jacob. “I wouldn’t have made it if it weren’t for Ann supporting me during those difficult months.”
Still, after seven months of the second chemo round, Jacob wasn’t able to completely shake the disease as marked by the MRD (measurable residual disease). A fresh approach was crucial. In 2019 the hematologist in Oslo mentioned a new treatment in Bergen at the Haukeland University Hospital run by professor Bjørn-Tore Gjertsen. Jacob and Ann met with Bjørn to learn about the Advance II clinical trial, which used Mendus’ drug vididencel as a maintenance therapy for AML patients diagnosed with MRD.
“Hearing there was another option that could possibly work was fantastic news,” he said. “After two rounds of chemo and the horrible side effects, we were excited by this opportunity.”
The treatment consisted of a series of injections of vididencel, a drug designed to use the body’s own immune system to build up active, long-lasting immunity against tumor cells. The biggest side effect was redness and some swelling around the injection site for a few days, far easier to manage than the side effects from his months on chemo.
“Bjorn and his team were very kind to us, and the experience of going through this clinical trial was well worth it given the close attention they received. The MRD is gone and while I still struggle with effects from the earlier chemo and my lung and heart issues, my quality of life has improved,” Jacob said. “Ann and I exercise every day together, taking walks in the forest or at the gym. We take this day by day and with our first grandchild set to join us in August we have a lot to look forward to.”

